Soil pH is a crucial parameter that affects the availability of nutrients to plants and the overall health of soil ecosystems. Monitoring soil pH is essential for optimizing agricultural practices, understanding soil dynamics, and ensuring proper plant growth. Soil pH sensors play a vital role in this process by providing accurate and real-time measurements of soil acidity or alkalinity. In this article, we will explore the working principles, types, calibration, and applications of soil pH sensors.

Working Principles of Soil pH Sensors:
Soil pH sensors operate based on different principles, including potentiometric, colorimetric, and optical methods. Let’s delve into each principle:
a. Potentiometric Method:
Potentiometric sensors measure the electrical potential difference between a reference electrode and a pH-sensitive electrode inserted into the soil. The pH-sensitive electrode consists of a glass membrane or a solid-state material that responds to changes in hydrogen ion concentration. As the soil solution comes into contact with the electrode, a voltage is generated, which is converted into a pH reading.
b. Colorimetric Method:
Colorimetric sensors use indicators that change color based on the pH of the soil solution. These sensors typically consist of a test strip or a pad impregnated with a pH-sensitive indicator dye. When the test strip comes into contact with the soil, the indicator reacts with the soil’s pH and changes color. The color change is compared against a color chart or analyzed using a colorimeter to determine the soil pH.
c. Optical Method:
Optical sensors utilize light absorption or fluorescence properties to measure soil pH. These sensors contain a pH-sensitive dye that emits or absorbs light in response to changes in pH. The intensity of the emitted or absorbed light is measured and correlated with the soil pH using calibration curves.
Types of Soil pH Sensors:
There are several types of soil pH sensors available, each with its advantages and applications. Let’s explore a few common types:
a. Electrochemical Sensors:
Electrochemical sensors, based on the potentiometric principle, are widely used for soil pH monitoring. They are reliable, durable, and provide accurate measurements. These sensors require proper calibration and maintenance to ensure optimal performance.
b. Colorimetric Test Strips:
Colorimetric test strips are inexpensive and convenient for rapid pH measurements in the field. They are often used by farmers and gardeners to get a quick estimate of soil pH. However, they may not provide highly precise readings compared to more sophisticated sensors.
c. Optical Sensors:
Optical pH sensors offer non-invasive and real-time monitoring capabilities. They are commonly used in research and precision agriculture applications where continuous monitoring is required. Optical sensors can be integrated into soil probes or placed directly in the soil to measure pH changes over time.
Calibration of Soil pH Sensors:
Calibration is essential for accurate soil pH measurements. To calibrate a soil pH sensor, it is necessary to use standard buffer solutions with known pH values. The sensor is immersed in the buffer solution, and the reading is adjusted to match the known pH value. Multiple buffer solutions covering a range of pH values should be used for calibration to ensure accuracy across the entire pH scale.
Regular recalibration is recommended
to account for any drift or changes in sensor performance over time. Calibration frequency depends on the sensor type, environmental conditions, and manufacturer’s recommendations.
Applications of Soil pH Sensors:
Soil pH sensors find widespread applications in various fields, including:
a. Agriculture:
Monitoring soil pH is crucial for optimizing crop production. Different plants have specific pH requirements, and maintaining the appropriate pH range ensures nutrient availability and reduces the risk of nutrient deficiencies or toxicities. Soil pH sensors help farmers make informed decisions about lime or acid application, irrigation strategies, and fertilizer management.
b. Environmental Monitoring:
Soil pH is an indicator of soil health and ecosystem functioning. Monitoring pH levels in natural ecosystems, such as forests or wetlands, helps assess soil degradation, acidification, and the impact of pollution. It also aids in the conservation and restoration of native habitats.
c. Research:
Soil pH sensors are vital tools in scientific research to study soil processes, nutrient cycling, and the effects of climate change on soil pH. They enable researchers to monitor pH dynamics in different soil types, identify soil acidification trends, and investigate the impact of land management practices.
Conclusion:
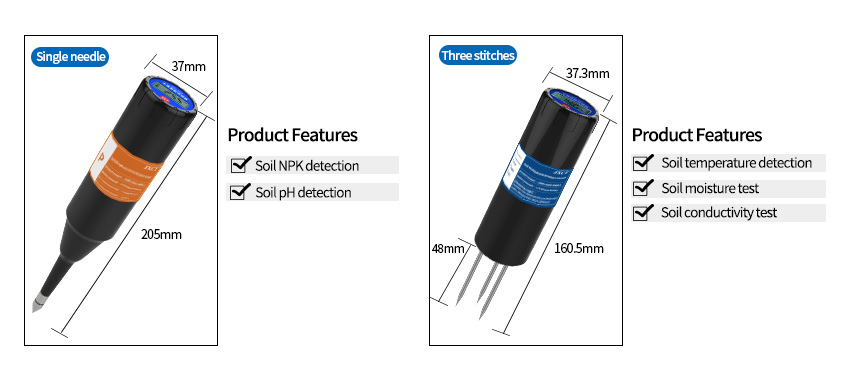
Soil pH sensors
play a crucial role in understanding soil characteristics and optimizing agricultural practices. By monitoring soil pH, farmers and researchers can make informed decisions about soil amendments, nutrient management, and environmental conservation. Understanding the working principles, types, calibration, and applications of soil pH sensors is essential for obtaining accurate and reliable measurements. By utilizing soil pH sensors effectively, we can promote sustainable agriculture, protect soil ecosystems, and ensure food security for future generations.